Metal electrolysis could support more efficient, eco-friendly processes for producing battery metals
“Here, we aim to establish some basic understanding to predict the efficiency of electrochemical metal production and metal-air batteries from examining computed thermodynamic barriers for the conversion between metal and metal oxides,” Yang Shao-Horn, one of the study’s authors, said in a media statement.
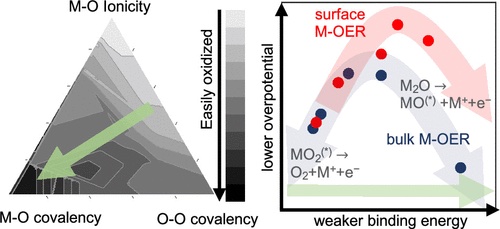
The work of uncovering the elementary steps involved in metal electrolysis, however, was challenging because it was unclear what those steps were. This meant that the researchers had to figure out how to get from metal oxide to metal and oxygen.
The analyses were conducted with supercomputer simulations. “It’s like a sandbox of atoms, and then we play with them,” co-author Michal Bajdich said.
More specifically, the team explored different scenarios for the electrolysis of several metals. Each involved different catalysts or molecules that boost the speed of a reaction. The map that emerged is essentially a guide for designing the best catalysts for each different metal.
Since they focused only on pure metals, the next step for the researchers is to see what happens in more complex systems involving multiple metals such as those where sodium and lithium are present.
The new atomic-level understanding of these reactions could not only help engineers develop efficient electrochemical routes for metal production but also design more efficient metal-air batteries because charging metal-air batteries also involves electrolysis.




