New developments improve dual-ion battery technology
In a paper published in Advanced Materials, the CAS researchers say they have provided a solution to what they call a “notorious issue” that causes the system to malfunction when the battery recharges and discharges.
According to Jiang Hongzhu, first author of the study, DIBs have attracted extensive attention due to their non-transition metal configuration, economy and environmental friendliness. However, practical implementation of the technology is nearly stagnant, mainly due to rapid battery failure during high-voltage cycling.
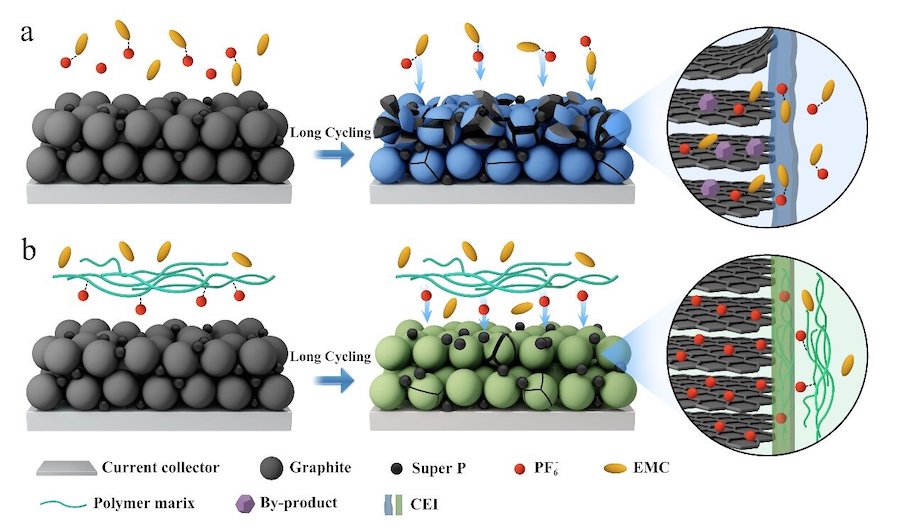
In DIBs, positively and negatively charged ions simultaneously move from the electrolyte to the opposite electrode. The “notorious issue” is that the solvent used in the electrolyte can insert into the graphite layers of the electrodes due to anion-solvent interactions.
“Eventually, this solvent co-intercalation results in graphite exfoliation and pulverization at high potential, especially in the widely used linear carbonate electrolytes,” Jiang said. She also noted that high-voltage cycling can lead to the oxidation of thermodynamically unstable electrolytes.
The scientist pointed out that previous strategies focusing on enhancing the stability of electrolytes have not effectively addressed the critical issue of solvent co-intercalation. Thus, to prevent this problem and electrolyte corrosion, she and her team needed to decouple the negatively charged anions from the solvent.
A viable approach to do so was to regulate the anion solvation structure by introducing another component that possesses stronger interaction with anions than carbonate solvents into the electrolyte.
The group decided to focus on hexafluorophosphate, an anionic component in lithium-ion batteries. They employed an important monomer containing quaternary ammonium motifs—which are positively charged—to develop a polymer electrolyte membrane that can selectively filter anions. This resulted in superb cycling stability with a 99% coulombic efficiency at high voltage.
“This strategy significantly inhibits solvent co-intercalation, as well as enhances the oxidation resistance of the electrolyte, ensuring the structural integrity of the graphite,” Cui Guanglei, co-author of the paper, said. “We believe facilitating the anion desolvation is crucial to ameliorate long cycling performance in DIBs.”




