‘Endangered metals’ no longer needed to produce hydrogen from water
In a paper published in the journal Nature Catalysis, the group behind the study explained that their goal was to address the issue of hydrogen extraction – or electrolysis – being an expensive and unsustainable process.
“This is primarily due to a lack of good catalysts,” lead researcher Ryuhei Nakamura said in a media statement. “In addition to being able to withstand the harsh acidic environment, the catalyst must be very active. If not, the amount of electricity needed for the reaction to produce a given amount of hydrogen soars, and with it, so does the cost.”
Nakamura explained that currently, the most active catalysts for water electrolysis are rare metals like platinum and iridium, which creates a dilemma because they are expensive and considered “endangered species” among metals.
According to the scientist, switching the whole planet to hydrogen fuel right now would require about 800 years’ worth of iridium production, an amount which might not even exist. On the other hand, abundant metals such as iron and nickel are not active enough and tend to dissolve immediately in the harsh acidic electrolysis environment.
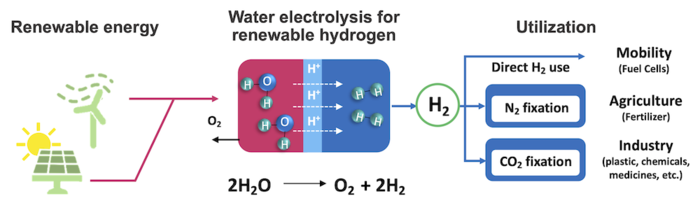
This is why in the search for a better catalyst, he and his team looked at mixed cobalt and manganese oxides. Cobalt oxides can be active for the required reaction but corrode very quickly in the acidic environment. Manganese oxides are more stable but are not nearly active enough. By combining them, the researchers hoped to take advantage of their complementary properties. They also had to consider the high current density needed for practical application outside the laboratory.
“For industrial-scale hydrogen production, we needed to set our study’s target current density to about 10 to 100 times higher than what has been used in past experiments,” co-author Shuang Kong said. “The high currents led to a number of problems such as physical decomposition of the catalyst.”
Eventually, the team overcame these issues by trial and error and discovered an active and stable catalyst by inserting manganese into the spinel lattice of Co3O4, producing the mixed cobalt manganese oxide Co2MnO4.
In their paper, the experts report that activation levels for Co2MnO4 were close to those for state-of-the-art iridium oxides.
Additionally, the new catalyst lasted over two months at a current density of 200 milliamperes per square centimeter, which could make it effective for practical use. Compared with other non-rare metal catalysts, which typically last only days or weeks at much lower current densities, the new electrocatalyst could be a game-changer.
“We have achieved what has eluded scientists for decades,” co-author Ailong Li said. “Hydrogen production using a highly active and stable catalyst made from abundant metals. In the long run, we believe that this is a huge step towards creating a sustainable hydrogen economy. Like other renewable technologies such as solar cells and wind power, we expect the cost of green hydrogen technology to plummet in the near future as more advances are made.”




