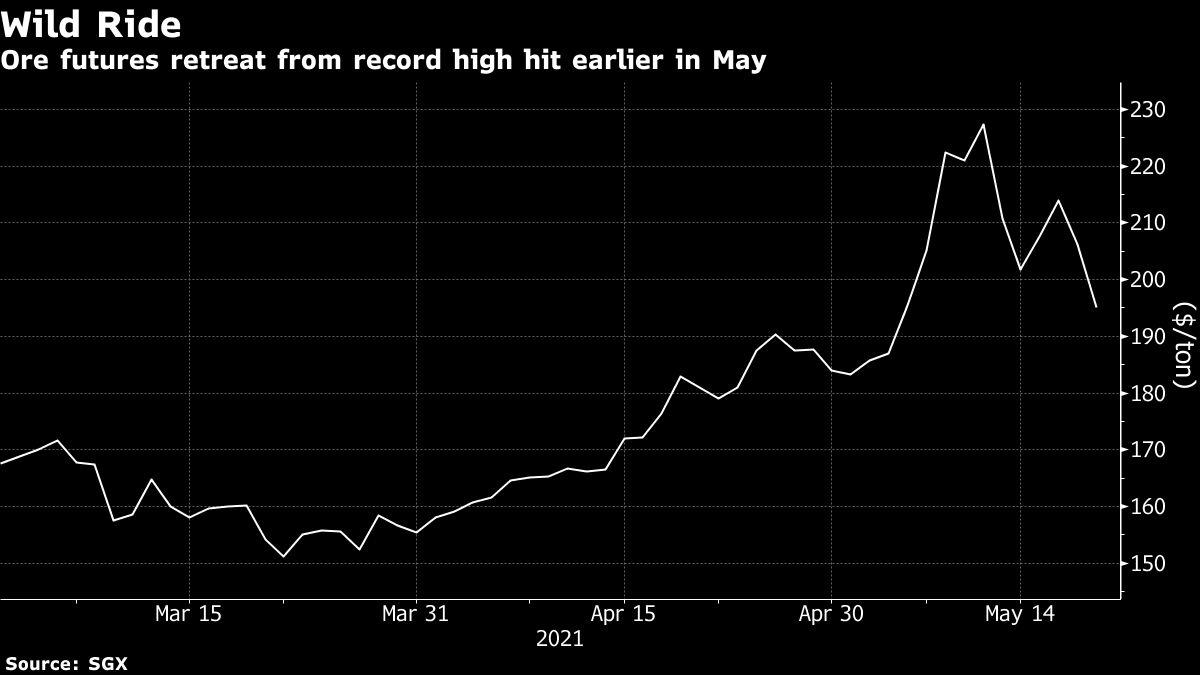
Giant New Iron Ore Mine May Aid China’s Push to Cool Prices
TipRanks
2 “Strong Buy” Stocks Trading at Steep Discounts
Whether markets move up or down, every investor loves a bargain. There’s a thrill in finding a valuable stock at low, low price – and then watching it appreciate in the mid- to long-term. The key here for investors is finding options in which the risk/reward combination will work toward long-term advantage. So, how are investors supposed to distinguish between the names poised to get back on their feet and those set to remain down in the dumps? That’s what the pros on Wall Street are here for. Using TipRanks’ database, we pinpointed two beaten-down stocks the analysts believe are gearing up for a rebound. Despite the hefty losses incurred over the past 52 weeks, the two tickers have scored enough praise from the Street to earn a “Strong Buy” consensus rating. Iovance Biotherapeutics (IOVA) The first stock we’re looking at, Iovance, is an immune-oncology company, working on tumor-infiltrating lymphocyte (TIL) therapies to be used in cancer treatment. The technology is designed to stimulate the patient’s immune system into attacking cancer cells. Iovance has an active pipeline, with six drug candidates under investigation – several for multiple applications. The lead candidate, lifileucel, is undergoing clinical trials as a potential treatment for melanoma and cervical cancer. The big news on Iovance in recent days directly concerns lifileucel. The drug, which has shown promise in early studies, is currently undergoing multiple Phase 2 clinical trials. Data from those trials is scheduled to be release in early June, and preliminary accounts suggest that Iovance has positive results to share. The other major development, however, was not as good for lifileucel or Iovance. The company this week acknowledged that the FDA has responded to its submission of assay data on lifileucel, and has requested additional information on potency prior to making a decision on the biologics license application (BLA). The BLA submission, which had been expected in 2H21, has now been pushed back to 1H22. Along with the regulatory hurdle, Iovance’s CEO, Maria Fardis, Ph.D., has announced that she is leaving the company. Her announcement came after the FDA feedback, and she will be succeeded on an interim basis by the company’s general counsel pending a search for a permanent CEO. On the news – of both the FDA feedback and the resignation of the CEO – Iovance shares tumbled nearly 40%. Covering the stock for Baird, analyst Colleen Kusy remains bullish on Iovance. Kusy rates the stock an Outperform (i.e. Buy), and his $55 price target suggests a 237% upside potential for the year ahead. (To watch Kusy’s track record, click here) “Determining an assay remains the rate-limiting step in completing the BLA submission for lifileucel in melanoma. Given additional data to be submitted to the FDA, expectations for BLA filing have been delayed from 2021 to 1H22. While the delay is disappointing, we view updated filing guidance as a bode of confidence in reaching an agreement on a potency assay with the FDA this year,” Kusy noted. The analyst added, “Based on the strength of the results to date, we continue to expect lifileucel will ultimately get approved in melanoma based on the current data package. Additionally, we see broad potential for lifileucel in multiple additional tumor types…” Overall, the analyst consensus on Iovance, even with the current events on the stock, is a unanimous Strong Buy, based on 11 recent reviews. The shares are priced at $16.33 after their recent slide, and the average target of $45.70 implies ~180% upside for the next 12 months. (See IOVA stock analysis on TipRanks) Applied Therapeutics (APLT) Staying in the pharma industry, next up we have Applied Therapeutics. The company is working on a pipeline of novel drug candidates designed to target ‘indications of high unmet medical need.’ In other words, Applied is working on new medications for serious, life-threatening, diseases that currently have no approved treatments. There are two compounds in APLT’s pipeline at present – AT-007, a treatment for Galactosemia (a rare hereditary inability of the body to convert the galactose in milk into glucose usable by the body), and AT-001, which is under investigation as a treatment for diabetic cardiomyopathy. AT-007 has recently completed a Phase 2 study on adult patients, and has a Phase 2 pediatric study still in progress. Data from the Galactosemia studies was presented at a professional forum in April, and an NDA is expected to be submitted by 3Q21. In addition to the Galactosemia studies, AT-007 is under investigation as a treatment for Sorbitol Dehydrogenase Deficiency (SORD deficiency), and the Phase 2 pilot study has already started. AT-001 is farther along in the pipeline, and the Phase 3 study was begun in 3Q19. The company expects that enrollment in the ARISE-HF global registrational study will be completed by the middle of this year. Efficacy data is expected in 2022. APLT shares peaked in February, and have been slipped since then, declining approximately 70%. The slide coincides with a public offering of 3.45 million shares, at a price of $23 each. The sale netted $74.4 million in proceeds for operations. After the stock sale Applied Therapeutics reported having $148 million in cash available as of the end of 1Q21. Given the company’s cash burn last year of $82 million, this gives APLT a cash runway of 22 months, or until January 2023. Among the bulls is Baird analyst Brian Skorney who notes the major pipeline projects as positives. “We continue to be encouraged by management’s progress, as the galactosemia NDA filing remains on track for no later than 3Q21, a Phase 2 pilot study in SORD deficiency has been initiated, and the ARISE-HF study in diabetic cardiomyopathy is expected to complete enrollment in mid-2021 (also on schedule). Moving forward, we see the upcoming galactosemia review as the next major catalyst for shares,” Skorney opined. To this end, Skorney rates APLT shares an Outperform (i.e. Buy), and his $35 price target implies it has room for 134% growth over the next 12 months. (To watch Skorney’s track record, click here) Overall, this is another biotech with unanimous approbation from the Street’s analysts; APLT has 3 recent positive reviews for a Strong Buy consensus rating. The $38.50 average price target suggests a robust 157% one-year upside from the current trading price of $14.96. (See APLT stock analysis on TipRanks) To find good ideas for beaten-down stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights. Disclaimer: The opinions expressed in this article are solely those of the featured analysts. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment.




